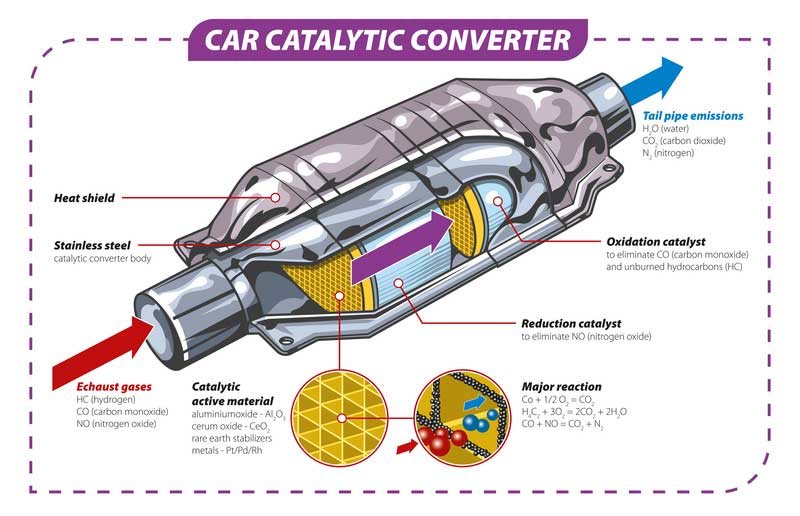
Catalytic converters do great work on harmful substances such as hydrocarbons, nitrogen dioxide, nitric oxide, and carbon monoxide coming through the car’s exhaust.
Such toxic substances are converted to less dangerous substances(such as water vapor and carbon dioxide)-inside the catalytic converters through chemical reactions.
But did you ask yourself what makes catalytic converters have such ability and what is made of? If you ever ask yourself such a question, worry not, as this article covers what you need to know about the metals making up the catalytic converters.
There are three common particular metal components used to design catalytic converters rhodium (Rh), Palladium (Pd), and platinum (Pt). Each metal has its unique role but let’s major on Palladium (Pd) today. The role of Palladium is to convert most harmful products such as carbon monoxide to less dangerous before being exposed to the environment.
With Palladium’s great work, do you know how much is in the catalytic converter?
Averagely, there are around 2 to 7 grams of Palladium in the catalytic converter. However, the palladium quantities vary with the type of catalytic converter model.
Some catalytic converters are small, and others are large, although the amount of Palladium used in catalytic converters will depend on its role.
The other two metal components, Platinum, and Rhodium are also rationed to fit the amount of Palladium available. In every 2 to 7 grams of Palladium, there are 1 to 2 grams and 3 to 7 grams of Rhodium and platinum, respectively. These metal components are so valuable, and it is the main reason why catalytic converters are expensive.
Contents
Why use Palladium with Platinum and Rhodium in designing catalytic converters?
Naturally, Palladium has high potential in improving oxidation. As well, Platinum increases the oxidation rate. Rhodium can reduce nitrogen oxides and is useful in making hydrogenation, acetic acid, and nitric acid reactions.
More ammonia is formed through reduction conditions when either Palladium or Platinum is combined with Rhodium. The chemical reactions occurring from the combination of these metals create conditions helpful in decreasing the conversion of nitric oxide under oxidizing surroundings.
In this case, when Palladium, Platinum, and Rhodium are used to make catalytic converters, toxic pollutants cannot escape the surroundings and cause harm.
For example, more than 89% of harmful substances are converted by Palladium in the catalytic converters to substances less harmful. This is done by converting toxic substances (such as nitrogen dioxide, carbon monoxide, hydrocarbons) to (less poisonous pollutants (such as carbon dioxide, nitrogen, and water vapor.)
The price of Palladium in the market today
If you ever wondered why catalytic converters are costly, it’s because of the materials used to make them, such as Palladium, platinum, and Rhodium. For example, the higher the price if the catalytic converters have more palladium quantities.
However, the prices of such catalytic converters vary with the value of its material components in the market. For example, 1 gram of Palladium today costs $73 .75, and an ounce will cost you $2,130.00 on average.
These palladium prices continue changing due to market fluctuations, although other factors can affect the prices. For example, extracting Palladium from a catalytic converter is expensive.
Indeed, each process in Palladium is expensive-starting with leaching chemicals such as nitric acid and sulphuric acid; these chemicals don’t cost less in purchasing. After the leaching process, an impurity removing process ends up in the precipitation stage. The precipitation process results in dichloramine-palladium(II) or yellow palladium salt.
The salty substance is then reduced to metallic Palladium then the smelting process is done. However, the final stage, smelting metallic Palladium to metal attainment, is regarded as the most costly process.
More heat is required to melt the metallic palladium ore and extract a base palladium metal. Once all the procedures are followed, attaining 99.9% pure palladium metal is achieved, and the final product is ready for market.
Such a process of extracting Palladium from other substances makes it expensive. Cars that use petrol have excellent catalytic converters and are of high prices. Even considering the rate of cars being stolen today, the rate of petrol featured cars is higher than that of diesel cars. The most targeted things in those stolen cars are catalytic converters sold to extract Palladium.
Conclusion
Palladium is considered expensive and makes catalytic converters so costly; for example, 1 gram today costs around $73.75 on average. There are 2 to 7 grams of Palladium in the catalytic converter. However, the grams range from one catalytic to another and car models.
